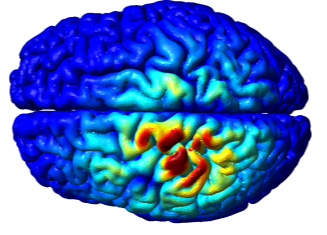
The Opitz Lab has many ongoing grants and research projects that support our goal of developing new methods for non-invasive brain stimulation. To see more details into our ongoing work, please visit our computational research, human studies, and animal research pages.